Mary Madison, RN, RAC-CT, CDP
Clinical Consultant – Briggs Healthcare
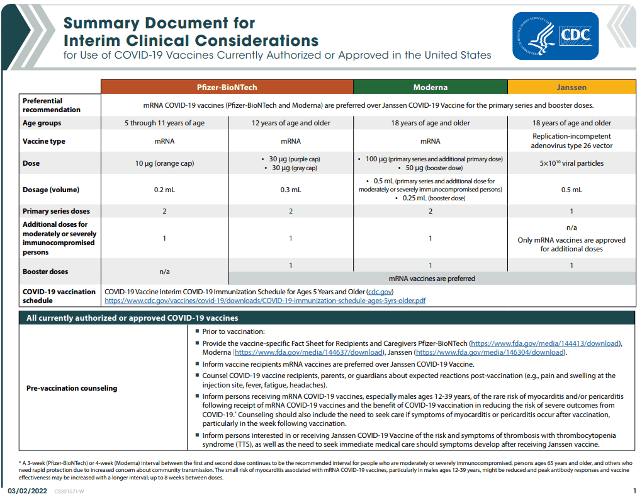
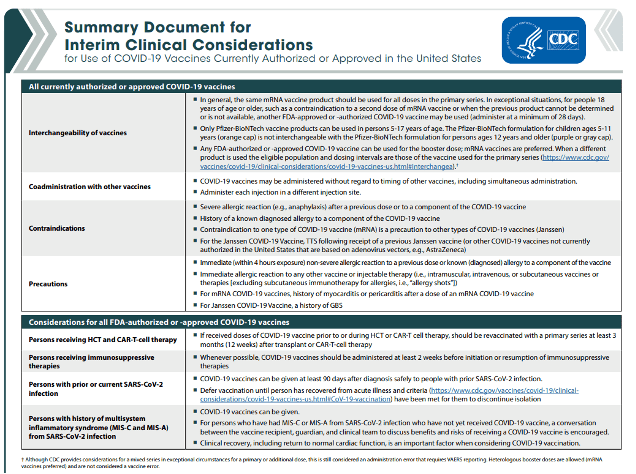
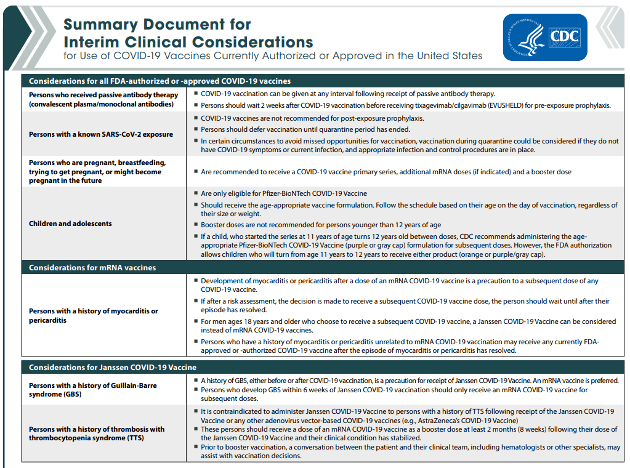
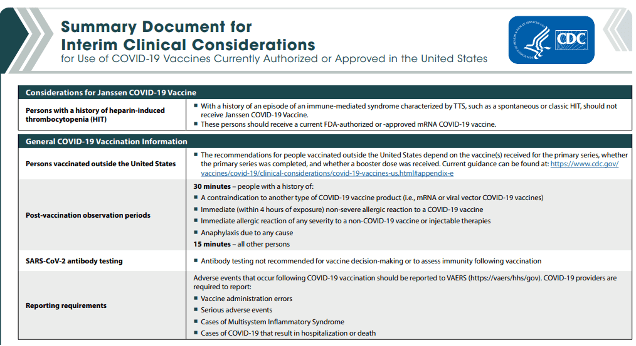
The CDC has updated its Summary Document for Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized or Approved in the United States. This is a 4-page document.