Mary Madison, RN, RAC-CT, CDP
Clinical Consultant – Briggs Healthcare
Today, September 26, 2022, CMS issued QSO-22-25-CLIA – same title as this blog.
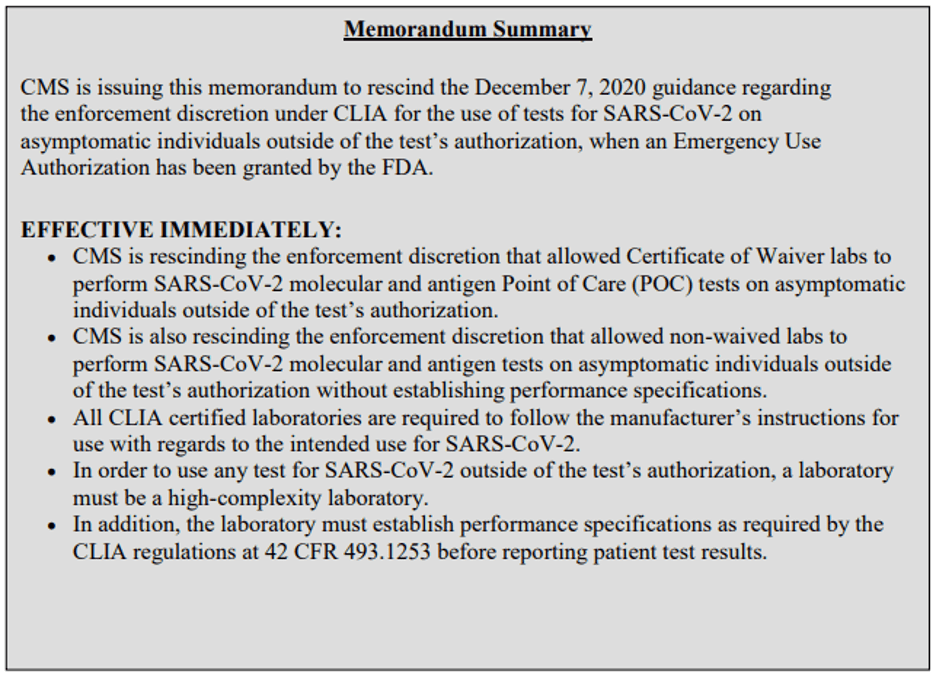
CMS issues this (3-page) memorandum to rescind the enforcement discretion under CLIA related to the use of tests on asymptomatic individuals outside of the test’s instructions for use. As home testing is now widely available and tests can be provided within the parameters designed to ensure accuracy, the original justification supporting this policy is no longer compelling.
All CLIA-certified laboratories are required to follow the manufacturer’s instructions for use with regard to the intended use for SARS-CoV-2. Under the CLIA regulations, in order to perform testing, a facility must be a CLIA-certified laboratory that meets applicable regulatory requirements appropriate for the complexity designation of the test. Any test for which the instructions for use have been modified becomes a high-complexity test.
If a test is modified, the laboratory must establish performance specifications as required at §493.1253 (b)(2). It is the laboratory director’s responsibility to ensure that the procedures used to establish performance specifications are adequate to determine the accuracy, precision, and other pertinent performance characteristics of the method (e.g., number of samples), and that the test method can provide the quality of results required for patient care. You may access the CLIA regulations at CLIA Regulations.
CLIA surveyors will cite a facility if it performs SARS-CoV-2 molecular and antigen POC tests on asymptomatic individuals outside the test’s authorization. Additionally, CLIA surveyors will cite a facility when tests are performed in such a manner without establishing performance specifications.
For questions or concerns relating to this memorandum, please contact LabExcellence@cms.hhs.gov.
